ORANJESTAD, Aruba – A drug importer notified the Health Inspectorate of Aruba that some batches of the drugs Losartankalium, Valsartan, and Irbesartan were recalled.
These batches were recalled from the pharmacies in Aruba because of contamination with the AZBT. One of the local importers in Aruba confirmed the import of the contaminated batch. This importer took the necessary action of informing all pharmacies. Consequently, the pharmacies removed these batches and are contacting the patients that received these medicines.
What are the medicine used for?
These are drugs prescription drugs for high blood pressure and heart disease patients. In Aruba, these drugs are available at pharmacies with a doctor’s prescription.
What happened?
The recall is due to contamination with AZBT, which may increase the risk of developing cancer.
It is essential to state that the contamination took place during the production process of the medicine and was not preventable. However, the quality control on drugs is very rigorous and the accepted contamination limit is very strict. Therefore, the chance that someone can get cancer by taking these medicines is very low. The amount of AZBT in these medicines is minimal. It means that the probability of getting cancer from taking these contaminated medicines (Losartankalium, Valsartan, or Irbesartan is 0.000001percent, thus practically zero percent.
What can the patient do?
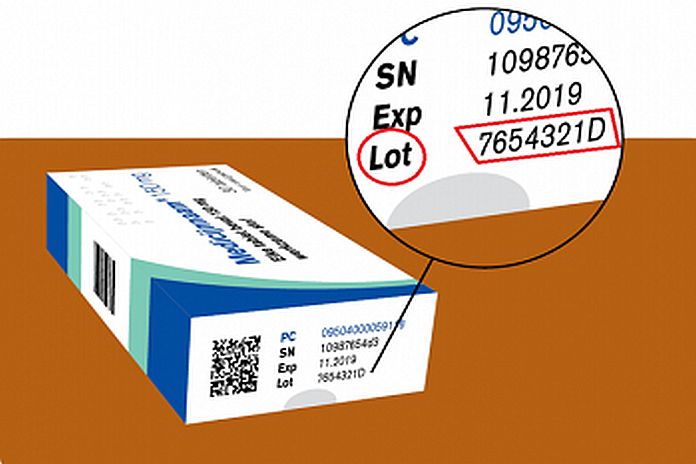
The pharmacy will contact the patients but Drs. Roselynn Angela, a Drug inspector of the Health Inspection of Aruba, urges everyone using these drugs, Losartankalium, Valsartan, and Irbesartan, to control the charge/lot number on the medicine package that is on the recall list. The charge/ lot number indicates the medicine batch and you can find it on the side of the medicine package (see the accompanying picture for an example). Only drugs packages with the charge number listed on the recall list must be returned to the pharmacies. You can find the recall list here. Hence, the charge number on the medicine must match the charge number on the list. Therefore, if the charge number on the medicine package matches the charge number on the list, the patient must contact his/her pharmacy.
The Drug Inspector is aware that many patients have questions or worries. However, she requests all patients, if they have drugs that fall under the list of the contaminated medicines and did not receive as yet a different drug from their pharmacy, to continue using this drug as prescribed. The risk of stopping using the drug without taking another high blood pressure medicine is considerably higher than the risk of the contamination. Patients with medicines that do NOT fall under the recall list do not have to do anything.
If you have any questions, you can also consult your family physician or pharmacy. The Inspectorate of health Aruba will inform the public of any change in due time.
Below you can find the letter of the pharmaceutical company and the complete recall list.