PORT OF SPAIN, Trinidad – The paediatric formulation of the Pfizer COVID-19 vaccine is not yet listed under the World Health Organization’s(WHO) Emergency Use Listing (EUL)
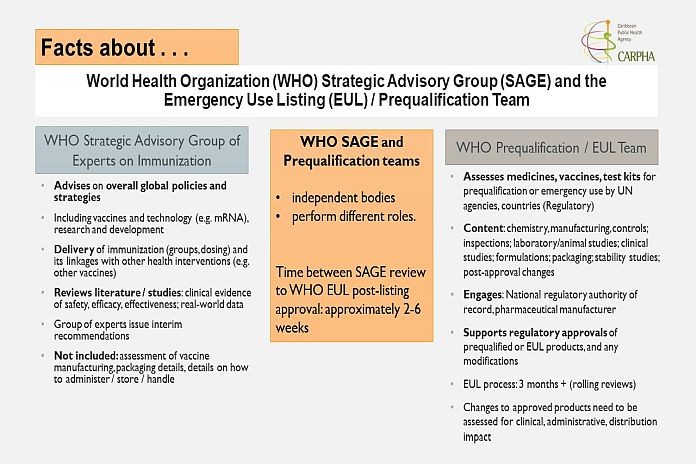
The WHO Strategic Group of Experts (SAGE) that advises on Immunization has recommended the use of the vaccine in children 5-11 years of age. However, the approval of the product in full comes from a different group of experts called the WHO EUL Prequalification Team.
This Prequalification Team looks at all aspects of the vaccine including:
- The composition;
- How it is made and where;
- How it is given (dispensed or administered);
- How it is packaged, and how it is to be stored.
This full product assessment is critical as it helps countries to be assured of the final vaccine product that may be registered in the country and used in children
CARPHA recognizes the WHO EUL procedure and collaborates with WHO Prequalification team to develop summaries and recommendations for vaccines listed under the EUL.
This is consistent with public presentations made by Dr Joy St John, the executive director of CARPHA and Dr Rian Extavour, programme manager, CARPHA Caribbean Regulatory System (CARPHA-CRS).
In that regard, at this time, CARPHA-CRS has not yet recommended the paediatric formulation due to the absence of confirmation of the new formulation by the World Health Organization (WHO) Prequalification team under the relevant Emergency Use Listing (EUL).