SWITZERLAND / USA – The World Health Organization’s (WHO) cancer research agency on Friday classified the commonly used artificial sweetener aspartame as “possibly carcinogenic to humans”, although another UN committee reaffirmed that there was a safe daily level of consumption.
The joint assessment from WHO’s International Agency for Research on Cancer (IARC), and the Joint Expert Committee on Food Additives (JECFA), which is part of WHO and the Food and Agriculture Organization, represents the first public intervention by the UN health agency on the widely used sweetener.
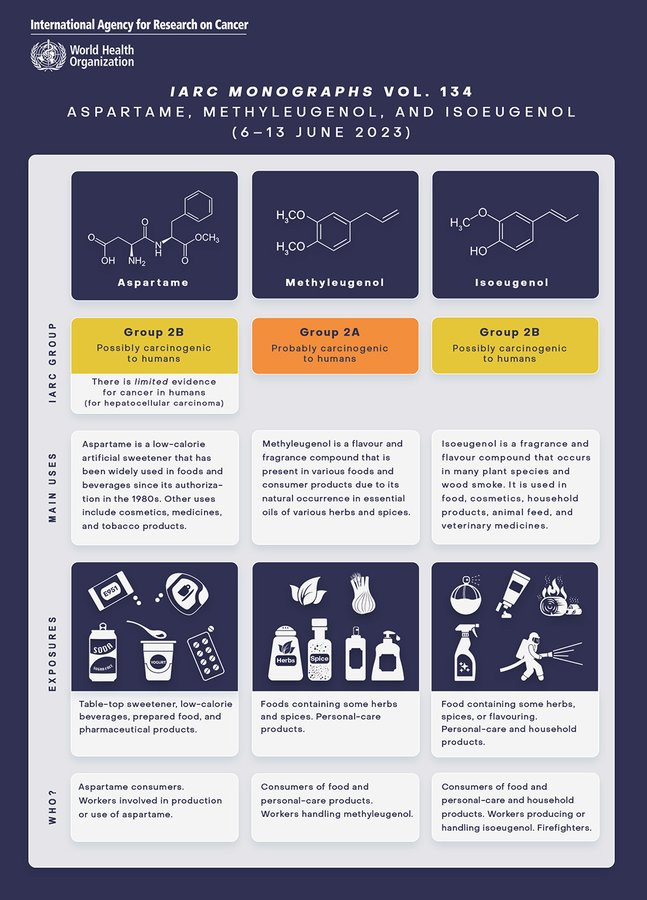
Aspartame has been used in multiple food and beverage products since the 1980s, including diet drinks, chewing gum, ice cream and other dairy products such as yoghurt; breakfast cereals, toothpaste, and medications such as cough drops and chewable vitamins.
The new assessments mark the first public evaluation of the sweetener by IARC, and scientists cite “limited evidence” that it could cause cancer.

More study needed
Dr Francesco Branca, Director of the Department of Nutrition and Food Safety at WHO said the assessments “have indicated that, while safety is not a major concern at the doses which are commonly used, potential effects have been described that need to be investigated by more and better studies.”
He reminded that one in six people dies from cancer: “Science is continuously expanding to assess the possible initiating or facilitating factors of cancer, in the hope of reducing these numbers and the human toll”.
The two bodies conducted independent but complementary reviews to assess the potential carcinogenic hazard and other health risks.
Safe consumption levels
JECFA concluded that it continues to be safe for a person to consume a substantial quantity of aspartame each day. An adult weighing around 70 kilogrammes (150lbs) would need to consume more than 9-14 cans of soft drinks to go beyond the recommended intake, assuming there was no intake from other sources.
“IARC classified aspartame as possibly carcinogenic to humans (Group 2B) on the basis of limited evidence for cancer in humans (specifically, for hepatocellular carcinoma, which is a type of liver cancer)”, a joint press release noted. “There was also limited evidence for cancer in experimental animals and limited evidence related to the possible mechanisms for causing cancer.”
“The findings of limited evidence of carcinogenicity in humans and animals, and of limited mechanistic evidence on how carcinogenicity may occur, underscore the need for more research to refine our understanding on whether consumption of aspartame poses a carcinogenic hazard,” said the IARC’s Dr Mary Schubauer-Berigan.
The IARC and JECFA evaluations were based on data collected from a range of sources, including peer-reviewed papers, governmental reports and studies conducted for regulatory purposes.
The studies have been reviewed by independent experts, and both committees have taken steps to ensure the independence and reliability of their work, they said.
FDA rejects conclusion
In a statement issued in reaction to the studies, the United States Food and Drug Administration (FDA), said that it disagreed with IARC’s conclusion that the studies support classifying aspartame as possibly carcinogenic.
“Aspartame is one of the most studied food additives in the human food supply. FDA scientists do not have safety concerns when aspartame is used under the approved conditions”, the statement said, adding that Health Canada and the European Food Safety Authority had both evaluated the sweetener and consider it safe at current permitted levels.
IARC and WHO said that they would “continue to monitor new evidence and encourage independent research groups to develop further studies on the potential association between aspartame exposure and consumer health effects.”





